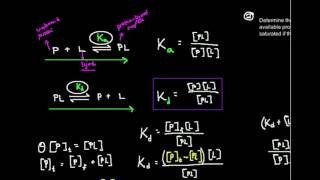
DISSOCIATION CONSTANT
Dissociation constant is the parameter which determines if an acid is weak or strong? The dissociation constant is the ratio of the dissociated ions (products) to the original acid (reactants). It is abbreviated as Ka. The dissociation constant of an acid can be determined by finding out the acid dissociates in water, when a acid is added to water, it dissociates in it.
Some of the hydrogen atoms from the acid are added to water, leaving behind the conjugate base of the acid. The process of transferring hydrogen atoms return to the conjugate base (reforming the acid) continues until the products and the reactants reach to the equilibrium.
Equilibrium is established when there are no changes in concentration of products and the reactants. Once the reaction reaches to equilibrium, we can determine the dissociation constant. Since it is a ratio of products to reactants, we can calculate it with a quotient.
The bracket around each group means the concentration of that molecule. it can be seen that the greater is the extent of dissociation, the greater will be the value of Ka or the stronger is the acid, the greater will be its Ka.
Thus, we can understand the significance of the values by describing following conclusions:
- The greater is the value of Ka, the stronger will be the acid and the weaker will be the base.
- The greater is the value of pKa, weaker will be the acid and stronger will be the base.
- The greater is the value of pH, the weaker will be the acid, and the stronger will be the base. For acids, ph<7 and for base, ph>7.
- The greater is value of R, the stronger will be the base and the weaker will be the acid.
- The greater is the value of pRy, the weaker will be the base and the stronger will be the acid.
- The greater is the value of pOH, the weaker will be the base and the stronger will be the acid. For base, pOH<7 and for acid pOH> 7.
APPLICATION OF DISSOCIATION CONSTANT
- Dissociation constant of drugs and pH of the environment of the absorption site is an important factor affecting the absorption of drugs through the membranes. This is very well understood by ph- Partition theory.
- The tendency of acids is to accumulate in basic fluids compartments while bases to acidic compartment. The reason is that acids become negatively electric charged in basic fluids, since they donate the proton. On the other hand, bases become positive electric charged in acid fluids, since they receive a proton.
- Since electric charge decrease the membrane permeability of the substances once an acid enters a basic fluid and becomes electrically charged, then it cannot escape that compartment with ease and therefore accumulates and vice-versa with bases. For a drug to cross the membrane barrier it must normally be soluble in the lipid material of the membrane and it has to be soluble in the aqueous phase as well to get out of the membranes.
- Many drugs have polar and nonpolar characteristics or are weak acids or bases. For drugs which are weak acids or bases; the pRa of the drugs, the ph of the Gl tract fluid and the ph of blood stream wil control the solubility of the drug and thereby the rate of absorption through the membranes lining of the Gl tract.
- pH of the absorption site decides the ratio of the ionized and unionized drug. pKa of the drug is the pH where drug exists as 50% ionized and 50% unionized.
.png)













.jpg)







 a quick overview on Nobel Prizes
a quick overview on Nobel Prizes  SURFACE AND INTERFACE TENSION
SURFACE AND INTERFACE TENSION