Introduction
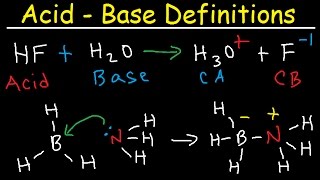
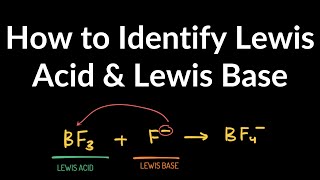
- Coordination compounds, also known as complexes, result from a donor-acceptor mechanism or Lewis acid-base reaction.
- These compounds involve the bonding of ligands (neutral molecules or anions) to a central metal ion through coordinate covalent bonds.
- Key points about coordination complexes:
- Ligands are Lewis bases or complexing agents.
- Metal atoms/ions are Lewis acids capable of accepting electrons from ligands.
- A coordinate covalent bond involves the donor atom supplying both electrons.
- If the complex carries a net charge, it is called a complex ion.
- Compounds containing coordination complexes are termed coordination compounds.
- Coordination compounds and complexes exhibit distinct properties compared to their individual components.
Ligands
- A ligand is an ion or molecule with at least one pair of electrons to donate to a central metal atom/ion.
- Ligands, also called complexing agents, can be neutral or negatively charged and act as electron donors to the central metal.
- Water is a common ligand, and its electron pair forms a bond with a central metal atom or ion (L → M).
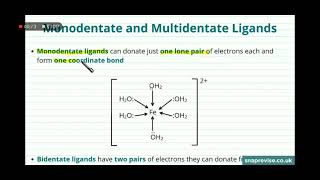
Monodentate Ligands
- Monodentate ligands have only one atom capable of binding to a central metal atom or ion.
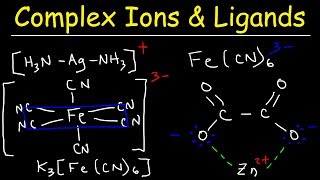
- Examples include H2O and NH3, and they can be neutral or charged (e.g., F-, Cl-, Br-, I-, CN-).
- The overall charge on a complex is the sum of the metal's oxidation state and the charges brought by each ligand.
- For instance, [Fe(CN)6]4- has a -4 charge.
Polydentate Ligands
- Polydentate ligands have more than one donor atom and are categorized based on the number of atoms they contain.
- Bidentate ligands (e.g., ethane-1,2-diamine) have two atoms capable of binding.
- Tridentate, tetradentate, pentadentate, and hexadentate ligands have three, four, five, and six donor atoms, respectively.
- Ambidentate ligands can bind in two possible places.
- Examples include NO2 and SCN.
.png)



















.jpg)
.png)






 a quick overview on Nobel Prizes
a quick overview on Nobel Prizes