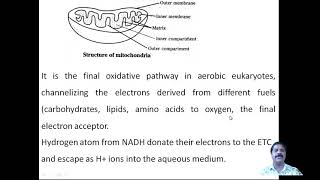
This post includes important one-liners of Biological Oxidation chapter from Biochemistry. This post summarises the chapter in short.
Biochemistry Important One Liners Questions On Biological Oxidation
1
The relation between the change of free energy (G), enthalpy (H) and entropy (S) is expressed by the equation
ΔG = ΔH – TΔS (T = Absolute temperature)
2
A negative sign of free energy indicates that the reaction is
Exergonic or spontaneous
3
The bonds responsible for a majority of high-energy compounds are
Phosphoanhydride bonds
4
The storage form of high-energy compound in invertebrates is
Phosphoarginine
5
A more negative redox potential represents a greater tendency to lose
Electrons
6
The electron transport chain is located in
Inner mitochondrial membrane
7
The prosthetic group present in cytochromes
Heme (porphyrin with iron)
8
The component of electron transport chain which can directly react with molecular oxygen
Cytochrome oxidase (cyt a + a3)
9
The site of ETC inhibited by cyanide
Cytochrome a + a3
10
Superoxide is converted to H2O2 by the enzyme
Superoxide dismutase
11
Name the compound with the greatest standard free energy.
Phosphoenolpyruvate
12
Which components of ETC possesses isoprenoid units
Coenzyme Q
13
The P : O ratio for the oxidation of FADH2 is
2
14
Inner mitochondrial membrane is impermeable to
OH– K+ H+
15
ATP synthase activity is associated with the mitochondrial enzyme complex
V