Molecules:
A molecule is in general a group of two or more atoms that are chemically bounded together by certain attractive forces.
For example – Two atom of hydrogen (H2) and one atom of Oxygen (O) combine with each other to form one molecule of water (H2O)
Atoms of the same element or a different element can join together to form molecules.
Molecules of Elements :
The molecules of an element are constituted by the same type of atoms.
For example – Helium (He) consists of only one atom while chlorine (Cl) consists of two atoms.
The number of atoms constituting a molecule is known as its atomicity. For example – helium is monoatomic and oxygen is diatomic.
1. Monoatomic – when an element is made up of only one atom. Ex – helium (He), all metals
2. Diatomic – when an element is made up of two atoms. Ex – oxygen (O2), all gases
3. Triatomic – when an element is made up of three atoms. Ex – ozone (O3)
4. Tetra-atomic – when an element is made up of four atoms. Ex – phosphorus (P4)
5. Poly- atomic – when an element is made up of more than two atoms. Ex – sulphur (S8)
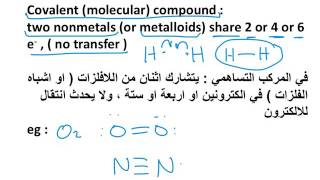
Molecules of Compounds :
Atom of different element combines together in definite or fixed proportion to form molecules of compounds.
For example – One atom of carbon (C) and two atom of Oxygen (O2) combine with each other to form one molecule of water (CO2).
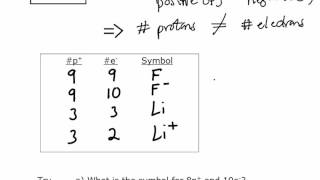
Ions:
Compounds are made up of metals and non-metals. These elements contain charged species, which are known as ions.
These are basically charged particles which can be both negatively (-) or positively (+) charged.
The element which has negatively charged ion is called an ‘anion’ and the element which has positively charged ion is called ‘cation’.
For example – NaCl , i.e., (Na+ ) ( cl –)
A group of an atom carrying a charge is called a polyatomic ion.