Download PDF – Dissociation Constant
How to Download Dissociation Constant PDF?
- Wait for 1 Minute until the download button unlocks.
- Click the Download Now button.
- Wait for 10 seconds while our system prepares your file.
- Your PDF will automatically download to your device.
- You can watch topic related videos below 👇 while waiting
⏳ Please wait 1 Minute to unlock your PDF.
🎥 Video Resources
Acid/Base Dissociation Constant

Acid/Base Dissociation Constant

Acid Dissociation Constant (Example)
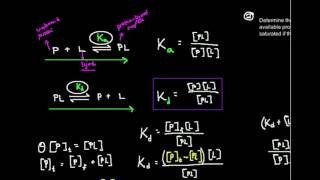
Kd, the Dissociation Constant: What is it?

pH of Weak Acids and Bases - Percent Ionization - Ka
How to Get PDF from Solotutes.com?
Getting your study material in PDF format from Solotutes is very simple:
- Open any post you want to download in PDF format.
- Just below the title, you will see a Download PDF button or link.
- Click on it and you will be redirected to this download page.
- Here, you will need to wait for 1 minute before the PDF button becomes active.
- Meanwhile, you can explore other resources available on this page such as related notes, quizzes, one-liners, or even watch relevant YouTube videos embedded here for better understanding.
- Once the timer ends, click on the Download Now button and your PDF will start downloading.
Why 1 Minute Wait?
⚡ To generate a clean, optimized, and printer-friendly PDF copy of Dissociation Constant.
💡 To support Solotutes with ads during this time, ensuring that our resources remain 100% free for students worldwide.
What You Will Get in Dissociation Constant PDF?
- ✔ A high-quality PDF of Dissociation Constant with proper formatting
- ✔ Easy to read offline on mobile, tablet, or desktop
- ✔ Great for revision, practice, and quick reference
- ✔ Additional learning support with related videos and resources linked on this page
- ✔ Supports our mission: “Knowledge for Everyone”
Why Choose Solotutes?
At Solotutes, we provide free access to notes, quizzes, MCQs, one-liners, and tests for multiple exams. By downloading Dissociation Constant in PDF format, you not only get valuable material but also become part of a growing community of learners. We regularly update our posts with videos, explanations, and extra content to help you understand topics better. Exploring these while waiting for your PDF ensures you don’t waste a single minute.
FAQs – Dissociation Constant Download
Q. Is this PDF really free?
✅ Yes! All downloads on Solotutes are completely free for students.
Q. Do I need to sign up?
❌ No, you don’t need to create any account. Just wait for 1 minute and click download.
Q. Can I share this PDF with my friends?
✔ Yes, please share and help others learn too.
Q. Can I access other resources while waiting?
👍 Yes, you can scroll down to check related quizzes, notes, and YouTube videos while your download is getting ready.
About Solotutes
Solotutes.com is dedicated to students preparing for competitive exams, schools, and universities. We provide free PDFs, quizzes, MCQs, notes, and one-liners to make learning easy and accessible. Our aim is to save your time, keep you motivated, and ensure you always have the best study material — anytime, anywhere.

